Wet Specimen Tutorial
- Fixative: Formalin Replacement (95% Ethanol works well!) or Formalin
- Holding Fluid: 70% Isopropyl Alcohol or 70-95%% Ethanol, sometimes combined with glycerin/propylene glycol to create neutral buoyancy or help with hydrating delicate specimens
- Glass or Plastic Jar (with tight fitting & sealing lid, metal will corrode, so sealing with silicone may be necessary with some lids and can help create a seal)
- Syringe + Needle (for larger specimens)
- RO/DI Water
- PPE (Gloves, Goggles, Respirator)
- Containers for Rinsing/Defrosting
- Posing Supplies (Stainless steel wire, fishing line, or plastic pieces, to hold parts of the specimen in place during the fixation process if you want them in a particular pose)
Step 1: Acquire a fresh or frozen specimen, defrost if needed. I like to place my frozen specimens in water to defrost because it speeds up the process quite a bit! You can soak them directly in RO/DI water, or use a plastic bag to "float" them in tap water. Do not use hot water as it can damage a specimen.
Step 2: Clean the specimen. This may not be needed in all situations, but when preserving aquatic animals, I like to rinse the specimen several times to remove any excess slime coat. However, be very gentle so you do not damage the specimen or remove scales (if present). Some other animals may have dirt, debris, and bodily fluids still on their coat/skin which can interfere with the preservation process. Be sure to remove as much of this as possible before preserving. RODI water is ideal for cleaning because it doesn't contain any chemicals that may interact with your preservatives and is osmotically neutral.
Step 3: Place the specimen in the initial preservative for fixation, injecting well with the preservative if needed. You can use:
- Humectant fluid: This will not technically "fix" your specimen but can work, I have used this in the past and still have very sturdy specimens I made with it, but I can't claim they will last as long as ones fixed with formalin/ethanol.
- Ethanol: This will fix smaller specimens less than 3" thick, less success with larger specimens but possible if injected very thoroughly! Denatured ethanol is the most common, however the denaturants can interfere with the preservation process and are not standardized. Everclear is often available at liquor stores and does not contain a denaturant, making it an ideal ethyl alcohol at 150-190 proof. (Ethanol is often denatured to make it unconsumable - many denaturing agents make the alcohol incredibly bitter and undrinkable/toxic)
- A formalin replacement like "Prefer": Not enough research to show efficacy, but potentially usable.
- Formalin: Very toxic, expensive to ship, and requires great care when working with, you must wear goggles and work in a well-ventilated area, but is the best fixative in my opinion with reliable and consistent results.
10% buffered formalin is the industry standard for most wet specimen preparations, and I definitely recommend using it over most other preservatives. Ethanol is the second-best industry standard, but it is very dehydrating and can fail on larger specimens, especially when denatured.
Any animal with a body part diameter of more than one inch should be injected. I use 18-25 gauge needles depending on the thickness of the specimen's skin/scales. Inject in every area that you can, the body cavity, musculature, and head being the most important as these areas are more prone to rotting. Inject until the specimen is somewhat bloated and the preservative begins to leak out.
Be sure not to inject air as this can cause the specimen to float. If air is injected, you can unscrew the needle on your syringe and use it to aspirate where you think the air bubble is. If you do not have access to a needle, you can make small, deep holes or slits in the specimen with a scalpel to allow the preservative to saturate the entire specimen and release bubbles.
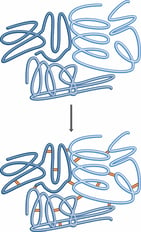
Fixation is the most important step of fluid preservation. Putting a specimen directly into isopropyl alcohol or hand sanitizer does not stop deterioration of the specimen over time. Isopropyl alcohol works well as a preservative and anti-microbial (killing bacteria and fungi that cause decomposition), but will not keep soft tissues stable, leading to a specimen falling apart eventually.
Fixation is the process of stabilizing proteins to make them stronger and not deteriorate/denature. It causes protein chemicals to bind (fix) to each other creating a strong matrix of cross-linked proteins, which in turn create very stable tissues. Image Source: National Diagnotstics
Step 4: Now it is time to wait. The specimen must soak in the initial preservative (fixative) for 1-2 weeks if it's small, and over a month if it is very large. Monitor your specimen as fixation time varies greatly depending on size/skin or hair texture.
Inverting/swirling the jar gently every few days is also helpful and ensures that the preservative is making its way into the entire specimen. After a few days, the specimen will begin to stiffen, so it's important that you have it positioned how you want it to stay forever. You can use certain "props" to pose the specimen, like pieces of plastic, fishing line, or stainless steel wire.
When a specimen is done with the fixation stage, it will be very different in terms of texture. You will be able to tell when it is done "cooking" by the stiffness and skin texture of a specimen. Formalin causes specimens to become quite rubbery and harder than they were before fixing. Ethanol will rehydrate and harden the specimen significantly. If you're unsure if a specimen is done, remove it from the fixative (wearing your PPE!!!!) and lightly press on the specimen to feel its texture. This may take practice to get just right, but if you feel any very soft/squishy parts, especially in the body cavity, let your specimen sit longer in the fixative.
Step 5: After soaking your specimen for a good while, it's time to switch out your initial preservative (fixative) for new 70% ethanol (if used as a fixative) or isopropyl alcohol.
Drain your jar and dispose of fluid (formalin is hazardous and should be discarded carefully, look into your local laws on this- I keep mine in 1 gallon plastic jugs wrapped in trash bags until I can dispose of it) alcohol can go down the drain, but be sure to dilute it heavily.
I usually rinse the specimen one more time in distilled water to remove any gunk and excess fluid that has accumulated. It is best to soak the specimen in distilled water for 24hr prior to switching to the holding fluid to ensure the preservative and holding fluid do not mix. I personally haven't had an issue skipping this step with smaller specimens, but I try to do this with any super delicate or large specimens when using formalin and then transferring to alcohol.
Occasionally, shriveling will occur if placed directly into a higher percentage alcohol from a fixative or lower percentage alcohol. To reduce this, acclimate your specimens to different alcohol concentrations very slowly via dilution or removal and concentration. I will occasionally use something like glycerin (which is very hydrating) in combination with alcohol on extremely delicate specimens like fish eggs, or even invertebrates, to create different effects like neutral buoyancy or increased hydration.
Fill your jar with alcohol and wait another few weeks (swirling the jar a bit just like during the fixation process). The alcohol will likely become discolored and can be changed however many times you'd like before putting your specimen on display. Red pigments or darker pigmented animals are more prone to staining the alcohol and will often require more alcohol changes.
Step 6: Once your specimen is done, you can leave it without changing the fluid again. The fluid may become discolored over time, but this is normal. Specimens used for scientific purposes usually never have their fluid changed as it is considered part of the specimen. However, if you don't like amber-colored fluid, you can swap your old alcohol out for fresh alcohol. If your lid isn't on tightly enough alcohol will evaporate, so monitor and refill if needed. It is always best to discard the old fluid completely and not "top-off" due to evaporation lowering the alcohol % over time creating osmotic imbalances.
Never leave your specimen in sunlight (this will bleach the specimen and increase degradation) or near a heat source (alcohol is highly flammable). Curved glass containers are also a fire hazard as they can create a magnifying effect, so be sure to keep those away from any strong light sources.
Sourcing Supplies
(I am not sponsored by any of these suppliers)
"Prefer" is a newer preservative on the market used as a formalin replacement. It is much safer to use than formalin, but I personally do not have experience with it. You can find it HERE
Formalin is highly toxic and more expensive to ship, however if you prefer to use this chemical, you can purchase it at 37% and dilute/buffer HERE, or 10% buffered formalin HERE
Ethanol is a great option as a holding fluid and at higher concentrations, a fixative for small specimens. You may need to dilute down to 70% with RO/DI water for holding purposes. You can purchase it (denatured) HERE or hop over to the liquor store for some everclear.
70% isopropyl alcohol (rubbing alcohol) is very easy to find at most grocery and drug stores, but can be purchased in larger quantities HERE
Glass jars can be found in many different places. I like to check out local thrift stores for more interesting pieces, but many grocery stores carry canning supplies as well. You can also buy many different sizes and shapes of glass jars online. I have found great 5-10ml jars on Amazon. I am sure many scientific supply companies have lots of bulk glassware as well.
Syringes and needles can be purchased at local pharmacies, as well as scientific supply companies. I tend to reuse needles until they are dull and have not had to purchase new ones in a very long time.
.png?width=300&height=367&name=Untitled_Artwork%20(1).png)
F.A.Q.
Can you preserve a specimen for me?
I would love to do this for people, but I am worried about dead animals getting lost in the mail and decaying. This may be a possibility in the future if I can figure out a reliable way to ship non-preserved specimens, but for now I recommend that you do it yourself. It is very inexpensive and easy to do.
Can I just put a specimen straight into a jar of alcohol?
Sometimes! I have had success preserving very small animals in just 70% isopropyl alcohol, especially small invertebrates like shrimp or crabs that consist of chitinous exoskeletons. I highly recommend preserving anything without an exoskeleton using the process above as fixation is the most important part of a long lasting wet specimen.
Can you put a specimen in resin?
Yes, but it's not a simple task. Any specimen that contains moisture can cause resin not to cure fully. I have seen many decaying specimens suspended in resin due to people not knowing any better. Plastination or mummification are the best ways to prepare specimens to fix in resin. I am currently looking more into mummification and will be making a tutorial soon~ but please don't try to mummify formalin-preserved specimens as they off-gas formaldehyde.
-1.png?width=800&height=1140&name=Untitled_Artwork%20(20)-1.png)